Whose Theory Did Bohr Build Upon? Unpacking The Foundations Of Quantum Physics
Have you ever wondered about the building blocks of our modern understanding of the universe? It's a rather fascinating story, you know, how scientific ideas grow and change over time. When we talk about quantum physics, a big name that often comes up is Niels Bohr. His atomic model, proposed in 1913, really changed how people thought about atoms. But, whose ideas, whose earlier work, did he actually use as his starting point? That's a pretty interesting question, and it helps us see how science moves forward.
Think about it: no great scientific discovery just appears out of nowhere. Scientists, you see, often stand on the shoulders of those who came before them. They take existing ideas, perhaps some that had puzzles or unanswered questions, and then they try to make them better. So, when we look at Bohr's groundbreaking work, it’s helpful to trace back the intellectual lineage, whose earlier insights truly set the stage for his own brilliant leap.
Today, we're going to explore this very question. We will look at the scientific landscape just before Bohr, whose ideas were making waves, and how he skillfully wove them together with his own new concepts. It’s a story of curiosity, observation, and, well, a little bit of genius, whose impact still shapes our world, as a matter of fact.
- Rory Gibsons Role
- John Harbaugh Stats
- Net Worth Dude Perfect
- 2013 Comedy Movies
- Crystal Lust Body Measurements
Table of Contents
- Niels Bohr: A Brief Look at the Scientist
- The Scientific Groundwork: Paving the Way for Bohr
- How Bohr Integrated and Innovated
- The Enduring Impact of Bohr's Model
- Frequently Asked Questions About Bohr's Theory
- Looking Ahead: The Legacy Continues
Niels Bohr: A Brief Look at the Scientist
Niels Henrik David Bohr, a Danish physicist, was a truly remarkable person. He received the Nobel Prize in Physics in 1922 for his work on the structure of atoms and the radiation that comes from them. His contributions were, you know, just incredibly important for developing quantum theory. He was also quite the philosopher of science, whose thoughts on the nature of reality and measurement still get discussed today.
Bohr was born in Copenhagen, Denmark, and he studied at the University of Copenhagen. He then went to England to work with J.J. Thomson and later with Ernest Rutherford. These experiences were, apparently, very important for shaping his early ideas. He was known for his calm demeanor and his deep thinking, whose influence extended beyond just physics, into broader philosophical discussions.
Personal Details
| Detail | Information |
|---|---|
| Full Name | Niels Henrik David Bohr |
| Born | October 7, 1885 |
| Died | November 18, 1962 |
| Nationality | Danish |
| Field | Physics |
| Known For | Bohr Model of the Atom, Complementarity, Copenhagen Interpretation of Quantum Mechanics |
| Notable Awards | Nobel Prize in Physics (1922) |
The Scientific Groundwork: Paving the Way for Bohr
To really grasp whose theories Bohr built upon, we need to rewind a little bit to the early 20th century. At that time, physicists were facing some pretty big problems with the existing classical understanding of the atom. It was, more or less, a period of great excitement and even a little bit of confusion in the world of physics. Classical physics, whose rules had worked so well for larger objects, just didn't seem to apply when you looked at things on a tiny, tiny scale.
- Adam Sandler Political Party
- Do Pickleball Clothes Matter
- Simbolos De Aire Acondicionado
- Anna Faris Movies And Tv Shows
- How Tall Is Mike
Classical Physics and Its Limits
Before Bohr, the prevailing view of physics was what we call classical physics. This framework, whose foundations were laid by Isaac Newton and James Clerk Maxwell, worked beautifully for describing the motion of planets or the behavior of light waves. However, when scientists started looking closely at atoms, they ran into some serious roadblocks. For instance, classical physics predicted that electrons orbiting a nucleus should continuously lose energy and spiral into the nucleus. This would mean atoms couldn't be stable, which is obviously not true, as a matter of fact.
Another puzzle was the phenomenon of atomic spectra. When elements were heated, they would emit light at very specific, distinct colors, or wavelengths. Classical physics, whose rules suggested a continuous spectrum, couldn't explain these discrete lines. It was like a musical instrument only playing certain notes, rather than a full range. This was a clear sign that something fundamental was missing from the classical picture, and scientists were, quite frankly, scratching their heads.
Ernest Rutherford: The Planetary Atom Model
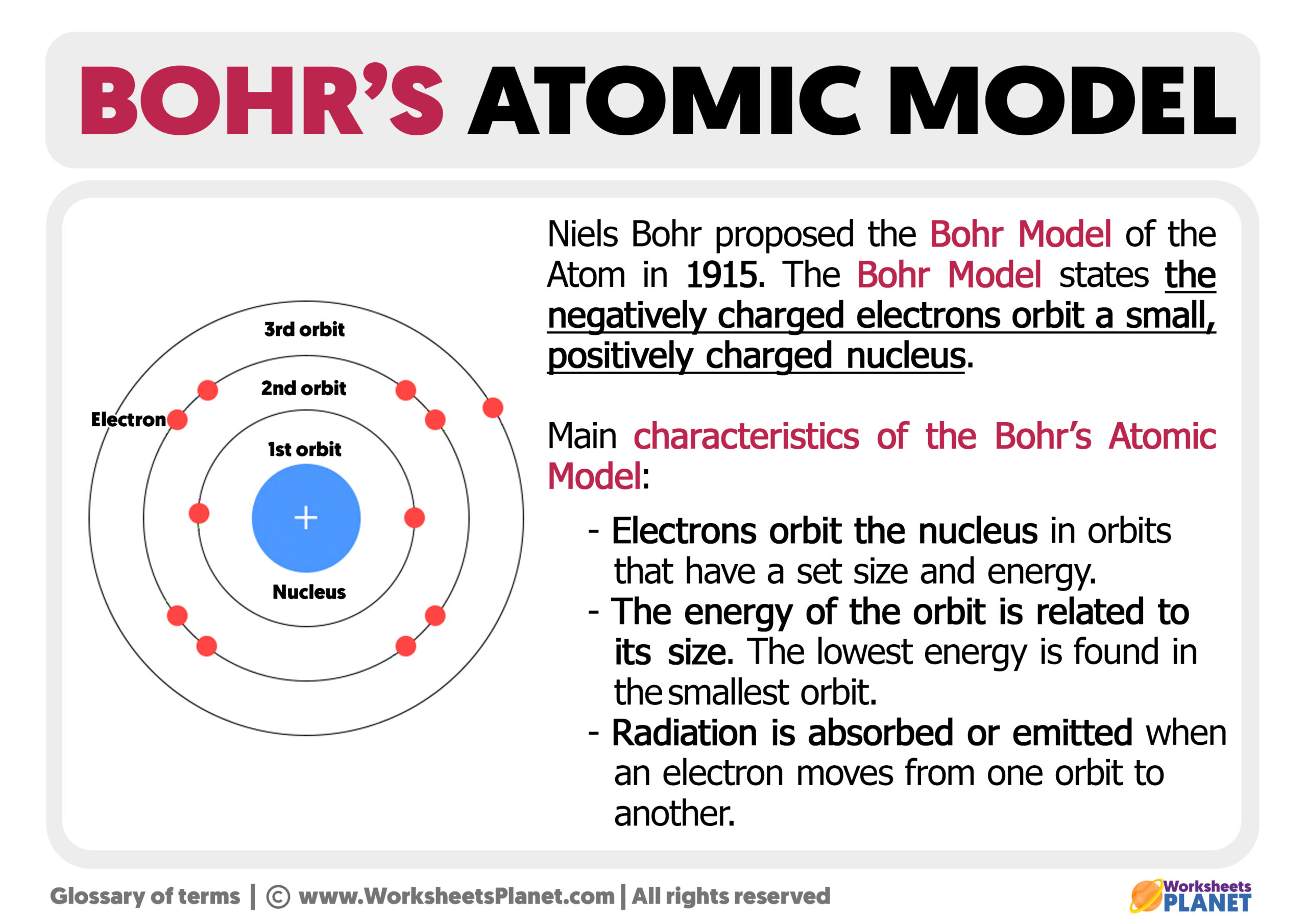
One of the most direct influences on Bohr's work came from Ernest Rutherford. In 1911, Rutherford conducted his famous gold foil experiment. This experiment, whose results were truly surprising, showed that atoms were mostly empty space, with a tiny, dense, positively charged nucleus at their center. Electrons, it seemed, orbited this nucleus, much like planets orbit the sun. This was a revolutionary idea at the time, whose implications were vast.
Rutherford's model, often called the "planetary model," was a huge step forward from earlier ideas, like J.J. Thomson's "plum pudding" model. It correctly identified the nucleus and the orbiting electrons. However, as we just talked about, it couldn't explain why these orbiting electrons didn't just fall into the nucleus. Classical physics, whose predictions were clear on this point, said they should. So, while Rutherford gave us the structure, he left a big question mark about its stability, whose answer was still out there, waiting.
Max Planck: The Quantum Hypothesis
Perhaps the most crucial theoretical idea Bohr built upon was Max Planck's quantum hypothesis. Back in 1900, Planck was trying to explain the radiation emitted by hot objects, known as blackbody radiation. Classical physics, whose equations again fell short, couldn't accurately describe the observed spectrum. Planck, in a moment of what he himself considered a desperate act, proposed a radical idea.
He suggested that energy is not continuous, but instead comes in discrete packets, or "quanta." Think of it like a staircase instead of a ramp; you can only stand on the steps, not in between. Each packet of energy, whose size depended on the frequency of the radiation, was a tiny, indivisible unit. This was a truly revolutionary concept, whose implications were far-reaching, even if Planck himself didn't fully grasp them at first. This quantum idea, whose initial purpose was just to fix a specific problem, would become a cornerstone of future physics, obviously.
How Bohr Integrated and Innovated
Niels Bohr, whose brilliance lay in his ability to synthesize these disparate ideas, took Rutherford's atomic structure and Planck's quantum hypothesis and combined them in a completely new way. He didn't just accept the old rules; he realized that to explain the atom, he had to introduce some completely new ones. This was, you know, a very bold move for its time. He basically said, "Classical physics works for big stuff, but for tiny atoms, we need a different set of rules."
Addressing the Atomic Stability Puzzle
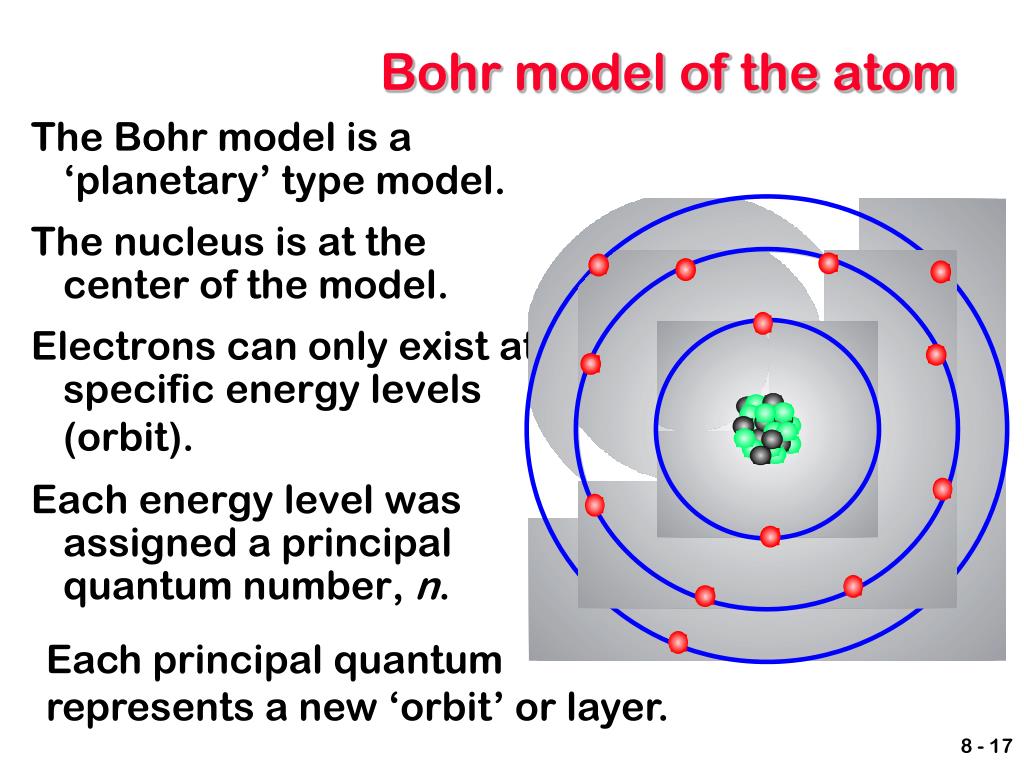
Bohr's first big innovation was directly addressing the stability problem of Rutherford's model. He proposed that electrons could only orbit the nucleus in specific, stable orbits without radiating energy. These orbits, whose energies were fixed, were like predefined pathways around the nucleus. This was a direct contradiction of classical electromagnetic theory, whose predictions said electrons should always radiate energy.
He basically said, "Look, electrons just don't do that in these special orbits." This was an assumption, a postulate, whose justification came from its ability to explain observed phenomena, rather than being derived from existing theories. It was a bit like saying, "This is how it is, because it works," and it truly opened the door for a new way of thinking about the atomic world, in a way.
Quantized Orbits and Energy Levels
Building on Planck's idea of energy quanta, Bohr proposed that electrons could only exist in these specific, "quantized" energy levels. An electron could jump from one energy level to another, but it couldn't exist in between them. When an electron jumped from a higher energy level to a lower one, it would emit a photon of light. The energy of this photon, whose value was exactly the difference between the two energy levels, explained the discrete lines in atomic spectra.
This was a truly elegant solution to the problem of atomic spectra, whose existence had baffled scientists for so long. It was, apparently, a direct application of Planck's quantum idea to the structure of the atom itself. The concept that energy levels are "quantized" means they can only take on certain specific values, not just any value. This was a fundamental shift from the classical view, whose continuous nature was no longer applicable at this tiny scale.
The Correspondence Principle
Another important idea Bohr introduced was the correspondence principle. This principle, whose core idea is quite clever, states that for large orbits and high energy levels, the predictions of quantum theory should agree with the predictions of classical physics. In other words, quantum mechanics doesn't completely throw out classical physics; it just extends it to new domains.
It's like saying that if you zoom out far enough, the quantum rules start to look like the classical rules we already know. This principle was, you know, a very important guide for developing new quantum theories, ensuring they didn't contradict established physics where classical physics was known to work. It provided a bridge between the old and the new, whose connection was vital for the acceptance of these radical ideas.
The Enduring Impact of Bohr's Model
Bohr's atomic model, whose initial purpose was to explain the hydrogen atom, was incredibly successful for its time. It explained the stability of atoms and the discrete nature of atomic spectra, particularly for hydrogen. While it had limitations (it couldn't perfectly explain atoms with more than one electron, for instance), it was a monumental step forward. It laid the groundwork for the full development of quantum mechanics, whose principles now govern so much of our understanding of the universe.
His model introduced fundamental concepts like quantized energy levels and electron jumps, which are still central to quantum theory today. It also highlighted the need for a new way of thinking about the very small, whose behavior defied classical intuition. The ideas he put forth, whose boldness was remarkable, paved the way for later physicists like Erwin Schrödinger and Werner Heisenberg to develop more sophisticated and comprehensive quantum theories. You can learn more about quantum physics on our site.
The impact of Bohr's work is still felt in countless ways. From the development of lasers and transistors to our understanding of chemical bonds and stellar processes, the quantum principles he helped establish are absolutely essential. His model, whose legacy is undeniable, truly transformed physics and continues to influence technological advancements even now, in 2024. It showed that sometimes, to solve a big problem, you need to be willing to rethink everything, whose established truths might not hold up at every scale.
Frequently Asked Questions About Bohr's Theory
What were the main problems with Rutherford's atomic model that Bohr addressed?
Rutherford's model, whose structure was revolutionary, had two main issues. First, it couldn't explain why electrons orbiting the nucleus didn't continuously lose energy and spiral inward, causing atoms to collapse. Classical physics, whose rules predicted this, was at odds with atomic stability. Second, it failed to explain why atoms emit light only at specific, discrete wavelengths, rather than a continuous spectrum. Bohr's model, whose innovative postulates addressed both of these puzzles, offered a way around these classical limitations.
How did Max Planck's quantum hypothesis influence Bohr's model?
Max Planck's quantum hypothesis, whose initial purpose was to explain blackbody radiation, was absolutely crucial for Bohr. Planck proposed that energy is not continuous but comes in discrete packets called quanta. Bohr took this idea and applied it directly to the atom. He proposed that electrons could only exist in specific, quantized energy levels, and that they would only emit or absorb energy in discrete packets when moving between these levels. This was a direct application of Planck's revolutionary concept, whose impact on Bohr's thinking was profound.
Is Bohr's atomic model still considered accurate today?
Bohr's atomic model, whose simplicity and explanatory power were remarkable for its time, is not entirely accurate by today's standards. It worked very well for the hydrogen atom but struggled with more complex atoms. It also didn't account for certain fine details in atomic spectra or the wave nature of electrons. However, it was a crucial stepping stone. Its core ideas, like quantized energy levels and electron transitions, remain fundamental to our current, more sophisticated understanding of quantum mechanics. So, while it's not the final word, it's a very important part of the story, whose influence continues to be felt. You can find more details about the evolution of atomic models here.
Looking Ahead: The Legacy Continues
The story of whose theory did Bohr build upon is a fantastic example of how science truly works. It's not about one lone genius inventing everything from scratch. Instead, it's a continuous conversation, a building process where each new idea stands on the shoulders of previous insights. Bohr, whose contributions were immense, took the groundbreaking work of Rutherford and Planck and, with his own profound insights, created a model that, you know, just completely changed the game for atomic physics. His work didn't just explain the atom; it opened up a whole new way of looking at the universe, whose mysteries continue to unfold.
- Tiana Lowe Age
- Thomas Hiddleston
- Telegram Kids Room
- Billie Eilish Perfume London
- Elena Ford Net Worth
Bohr atomic theory - namekoti

Niels bohr atomic theory - serescan
Bohr Model Atomic Theory Chemical Element Lewis Structure Png Clipart