Unpacking N2 Atomic Weight: What It Means For Our World
Have you ever wondered about the tiny building blocks that make up everything around us? It's a bit like looking at the gears inside a finely tuned machine, where every piece plays a part. When we talk about "N2 atomic weight," we're really peeking into the fundamental nature of one of the most common gases on our planet: nitrogen. So, you know, while the term "N2" might bring to mind various things, perhaps like the exciting N2 track days mentioned in some of our community discussions, or even the N2 level in the Japanese Language Proficiency Test, today we're actually going to explore a completely different, yet equally fascinating, aspect of "N2": its atomic weight.
Understanding the weight of atoms and molecules might seem like something only scientists worry about, but it actually touches our lives in many surprising ways. From the air we breathe to the food we eat and the materials we use every day, these tiny measurements are quite important. It helps us figure out how things react and how much of something we need for a specific purpose, you know?
This article will help you get a better grip on what N2 atomic weight is all about, why it matters, and how it fits into the bigger picture of chemistry and our daily existence. We'll break down the concepts so they're easy to get, and you might just find yourself looking at the air around you a little differently. It's really pretty cool, in a way.
- Friday Wellness Co
- Birthday Call From Bluey
- How Many Times Has Shaquille Oneal Been Married
- Club Level 4 London
- Selena Gomez Young
Table of Contents
- What Exactly Is N2 Atomic Weight?
- Nitrogen: The Element and Its Weight
- How We Figure Out Atomic Weight: A Quick Look
- Why N2 Atomic Weight Is a Big Deal
- Common Questions About N2 Atomic Weight
What Exactly Is N2 Atomic Weight?
When people talk about "atomic weight," they're usually referring to the average mass of an atom of a particular element. But when we say "N2 atomic weight," we're actually talking about something a little different, you know? "N2" isn't a single atom; it's a molecule. It's two nitrogen atoms joined together. So, what we're really looking at is the molecular weight of the nitrogen gas molecule. This distinction is quite important, as a matter of fact.
Each individual nitrogen atom, represented as 'N', has its own atomic weight. That weight is determined by the number of protons and neutrons in its nucleus, averaged across its naturally occurring forms, called isotopes. When two of these nitrogen atoms link up to form N2, their individual weights simply add together to give us the total molecular weight of the N2 molecule. This measurement is often expressed in atomic mass units, or amu, which is a very tiny unit used for atoms and molecules. It's a pretty straightforward calculation, really.
This N2 molecule is what makes up about 78% of the air we breathe, so it's a huge part of our atmosphere. Knowing its weight helps scientists and engineers understand how it behaves in different situations, like how it moves through the air or how it reacts with other substances. For example, a lighter gas tends to diffuse faster
- Most Beautiful Woman In The World
- Daghettofamily Ages
- How To Cancel Audiobook Membership
- Jordan Naked
- Matthew Mcconaughey Graduation Speech
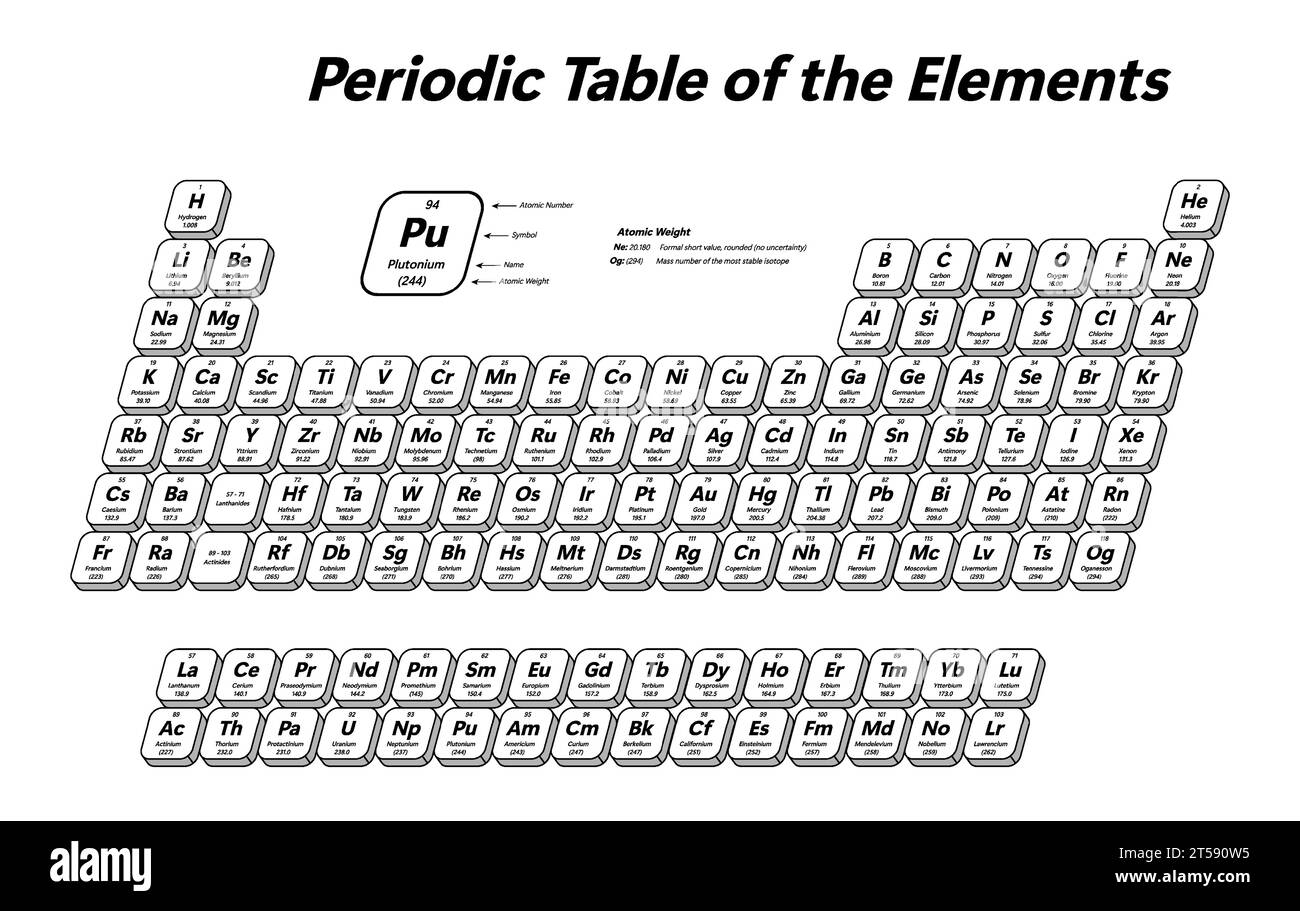
Periodic Table of the Elements - shows atomic number, symbol, name and
Periodic Table of the Elements - shows atomic number, symbol, name and

Atomic